🇮🇹 Leggi questo articolo in italiano 🇮🇹
IQOS is trending, especially among youngsters. Philip Morris International (PMI), the producer, claims it to be a healthier option to ordinary smoking. But is it really so?
The U.S. Food and Drug Administration (FDA) requires tobacco manufacturers and importers to report the levels of harmful and potentially harmful constituents (HPHCs) found in their tobacco products and tobacco smoke. HPHCs are chemicals or chemical compounds in tobacco products or tobacco smoke that cause or could cause harm to smokers or nonsmokers.
FDA published a preliminary list of 93 HPHCs in March 2012. This HPHC list focuses on chemicals that are linked to the five most serious health effects of tobacco use (cancer, cardiovascular disease, respiratory effects, reproductive problems, and addiction.)
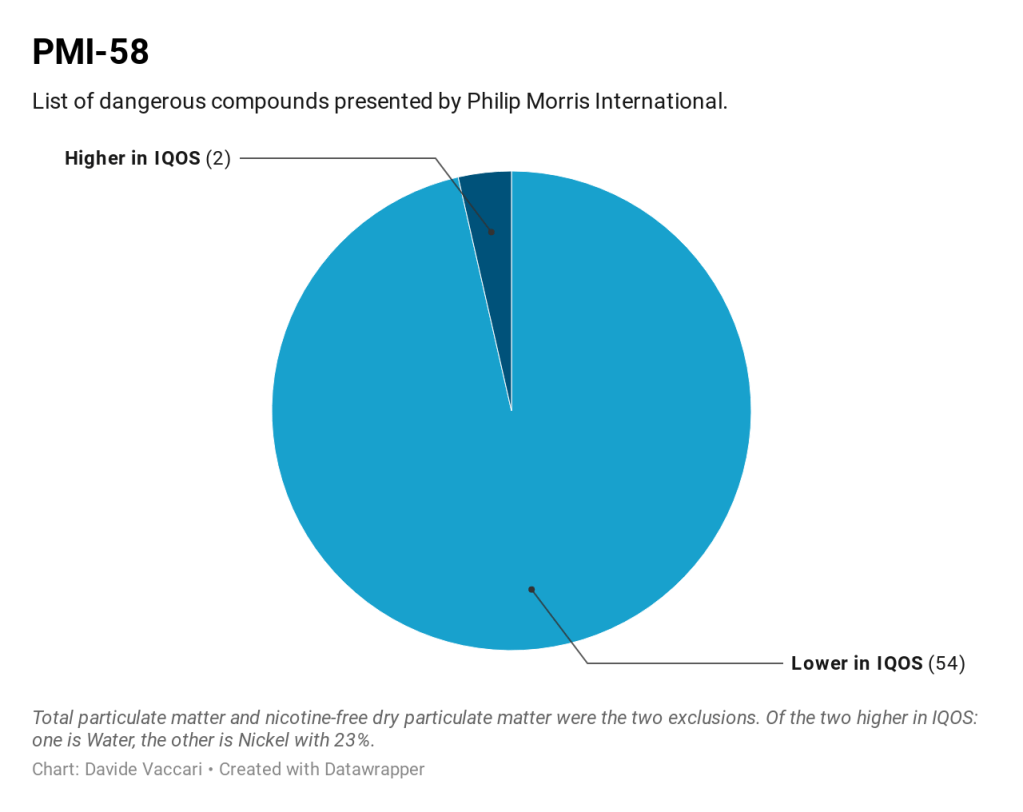
Philip Morris International (PMI) reported the levels of 58 constituents [Module 6.1.1] (which PMI refers to as ‘PMI-58’) in mainstream aerosol generated from IQOS and 3R4F reference cigarettes (3R4F is a standard reference cigarette, used throughout the tobacco industry and academic laboratories as a consistent and uniform test item for inhalation toxicology research. Developed by the University of Kentucky, Center for Tobacco Reference Products, it has been in use since 2006). The PMI-58 list includes 40 (43%) out of the 93 HPHCs on FDA’s list. The PMI-58 list included 18 additional constituents that do not appear on the FDA’s list of HPHCs, including water, total particulate matter, pyrene and nitrogen oxides. PMI concluded that the levels of HPHCs on the PMI-58 list were reduced by >92% on a stick basis and >89% on a normalized for nicotine basis for the regular tobacco stick, and >93% on a stick basis and >88% on a normalized for nicotine basis for the mentholated tobacco stick compared to 3R4F reference cigarette [Module 6.1.1, p. 45].
An addendum to the briefing document for the Tobacco Products Scientific Advisory Committee (TPSAC) meeting of the 24 and 25 January 2018, prepared by the FDA’s Center for Tobacco Products, presented additional data from PMI studies that showed higher levels of many substances in IQOS emissions compared with 3R4F cigarette smoke.
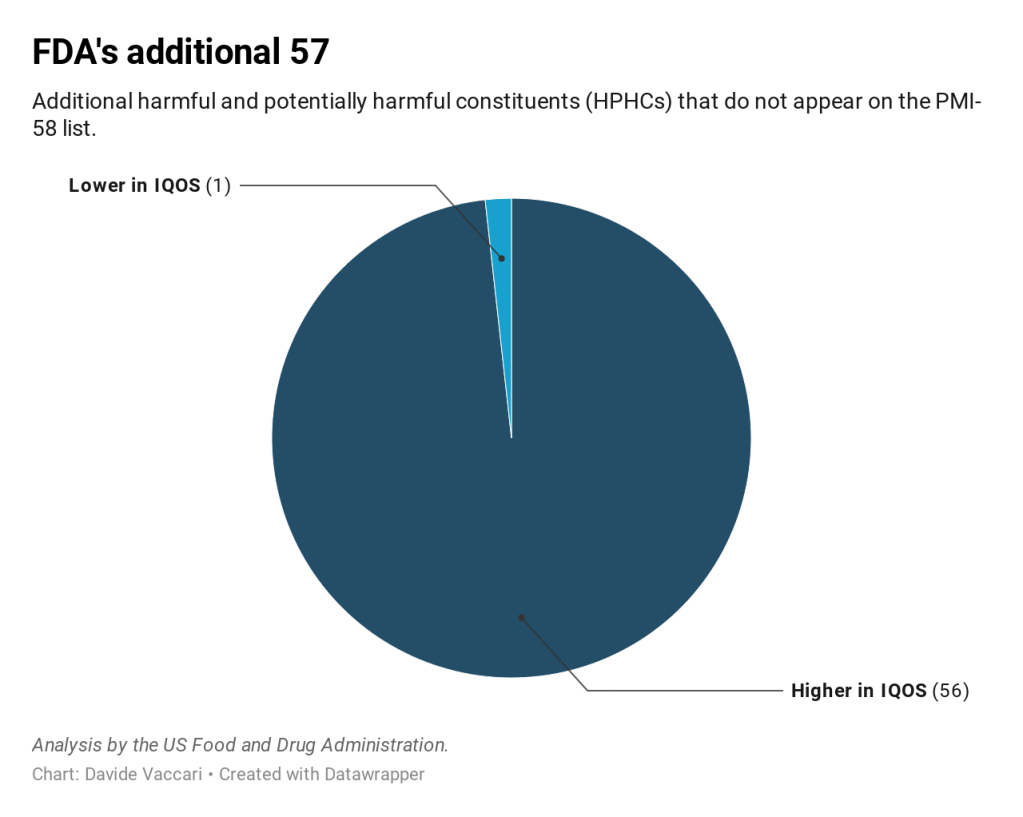
The addendum reported levels of 113 constituents, including 56 of the 58 constituents on the PMI-58 list (total particulate matter and nicotine-free dry particulate matter were the two exclusions) and 57 constituents that do not appear on the PMI-58 list. Fifty-six of the 57 non-PMI-58 constituents were higher in IQOS emission than in 3R4F smoke. Twenty-two of the non-PMI-58 constituents were at least 200% higher while seven were at least 1000% higher in IQOS emission compared with 3R4F mainstream smoke.
The purpose of this article is to show the percentual (%) change of compounds present in mainstream aerosol of Marlboro HeatSticks (IQOS) compared with 3R4F reference cigarette, matching the further findings of the mentioned addendum with the FDA’s HPCH’s list.
Data are publicly available. Anyone can access and download the original dataset of this work and is indulged to do so.
The compounds are ordered from the highest % presence in IQOS vs 3R4F to the lowest and categorized by their effects on human health.
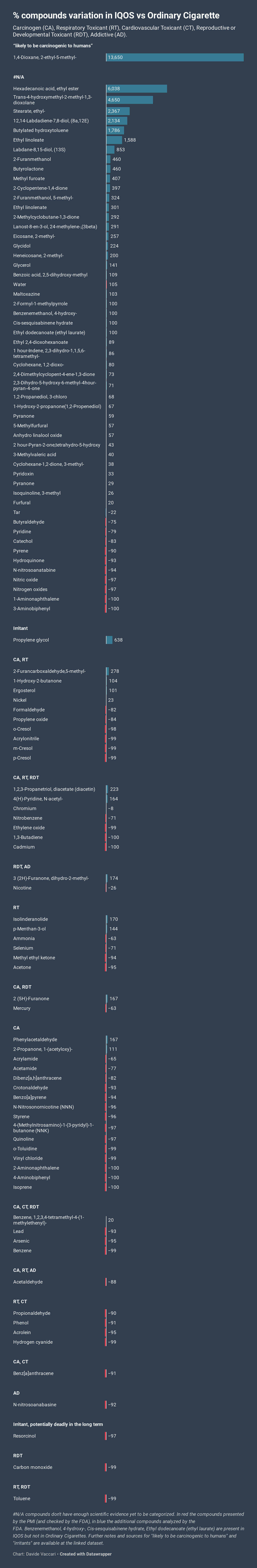
The results show that, while the 58 compounds presented by PMI were strongly decreased in IQOS smoke compared to the reference cigarette, there are many more constituents, considered harmful or potentially harmful by the FDA or other trusted medical sources, not reported by the Tobacco Company, that are present in PMI’s product in large-scale quantities in contrast to a normal cigarette.
In conclusion, there’re no scientific evidences about whether IQOS smoke would be less harmful than ordinary cigarette’s. Therefore, as for now, PMI’s claims are to be considered more as a (deceiving) marketing campaign rather than reliable scientific information.